PH value
The pH-value of leather
The pH of a substance means, at ten-logarithmic intervals, how acidic or basic this substance is. Distilled water is defined as having a pH of 7, so that a substance with a pH of 8 (such as seawater) is ten times more basic and a substance with a pH of 5 (such as coffee) is one hundred times more acidic. Coca Cola has a pH of 2 and is therefore one hundred thousand times more acidic than distilled water.
In the production of leather and leather care, the pH value is important as leather is produced with a pH of about 4.5 to 5.5. This pH ensures that the fat and tannins bound in the leather remain.
With the ageing of the leather, the acid-base ratio shifts to the alkaline (above pH 7), which makes the leather unstable. The leather darkens, becomes hard and brittle. The shift is due to age, humidity, sun, but also perspiration (skin, hair contact with car and furniture leather, sweat from feet in shoes (daily between 100-200 ml), hands in gloves or sweated collars of leather jackets).
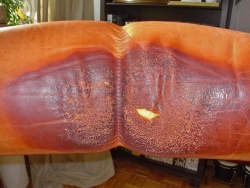
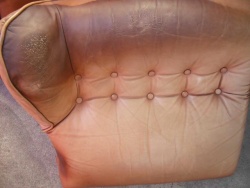
Typical damage due to skin and hair contact in furniture (head and armrests).
Steering wheels also react to skin contact with time. Due to the pH shift, the leather is also more susceptible to mould.
Skin-damaged car armrests and a shift knob.
Another reason is alkaline cleaners. Most household cleaners are alkaline, because they have a good cleaning effect. However, leather cleaners should not be alkaline, which is technically possible, but not considered by all manufacturers.
The leather care should also restore or maintain the acidic condition. Particularly, when old leather is contaminated with mould, acetic acid as a pH regulator is particularly suitable for removal and prevention. Also in the case of a restoration of leather, regulating the pH-value is just as important as the re-greasing to make the leather more resistant to environmental influences.
However, there is also a limit to how much acid a leather is capable of. Therefore, when regulating the pH, always be very careful not to damage the leather. A characteristic feature of book bindings, which can be observed in libraries, is red decay, when sulphur dioxide reacts with the air in the leather to become sulphuric acid, which irreparably erodes book covers. Sulphuric acid is one of the strongest acids and is far below the minimum pH-value of 3.5 for leather.
| Substance | pH-value | Type |
|---|---|---|
| battery acid | <0 | ↓ |
| stomach acid (sober stomach) | 1,0–1,5 | acid |
| lemon juice | 2,4 | |
| Coca Cola | 2–3 | |
| fruit juice of the shadow morello | 2,7 | |
| vinegar | 2,9 | |
| orange and apple juice | 3,5 | |
| wine | 4,0 | |
| sour milk | 4,5 | |
| beer | 4,5–5,0 | |
| acid rain | < 5,0 | |
| coffee | 5,0 | |
| tea | 5,5 | |
| rain (natural rainfall) | 5,6 | |
| mineral water | 6,0 | |
| milk | 6,5 | |
| water (depending on hardness) | 6,0–8,5 | |
| NEUTRAL | 7,0 | |
| human saliva | 6,5–7,4 | |
| blood | 7,4 | basic |
| sea water | 7,5–8,4 | |
| pancreas juice (intestinal juice) | 8,3 | |
| soap | 9,0–10,0 | |
| household ammonia | 11,5 | |
| bleach | 12,5 | |
| cement | 12,6 | |
| sodium hydroxide solution (caustic soda) | 13,5–15 |
Additional information















 a kotori web solution
a kotori web solution